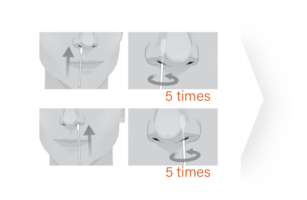
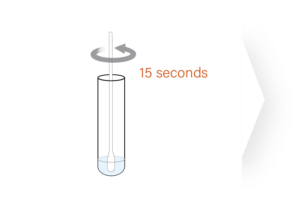
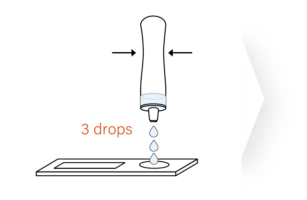
The ImmuView® COVID-19 Antigen Home Test is designed
to let you easily perform a COVID-19 self test.
FDA EUA – OTC COVID-19 Antigen Home Test for Ages 2 and Older: The ImmuView® COVID-19 Antigen Home Test is intended for the detection of SARS-CoV-2 (the virus that causes COVID-19). This self-administered test is for individuals ages 14 years and older. Adult collection is required for testing children ages 2-13 years old.